Built on CRISPR-Modified Chicken PGCs
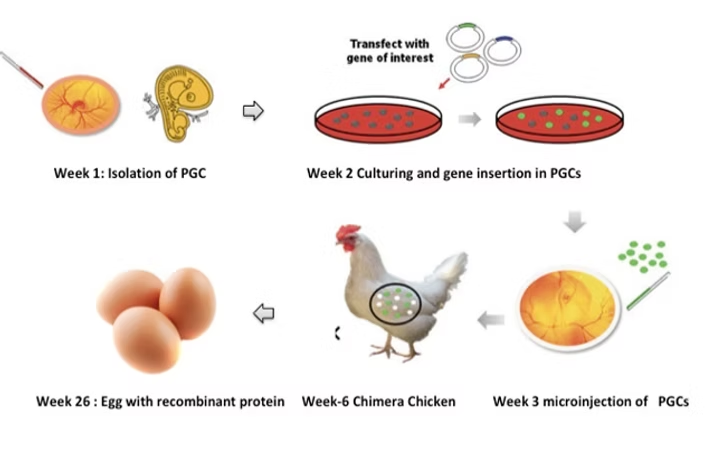
Primordial Germ Cells are the foundation of our innovative bioreactor system
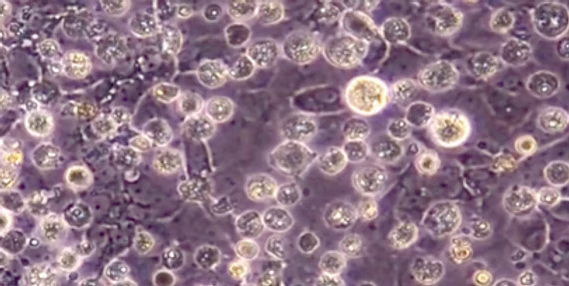
Primordial Germ Cells (PGCs)
Primary undifferentiated stem cells that differentiate towards gametes: spermatozoa or oocytes. PGCs serve as tools for storing high-quality genetic resources, wildlife conservation, and expression of recombinant proteins.
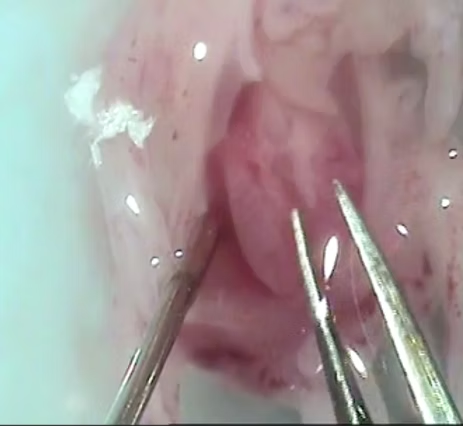
Gene Editing Capability
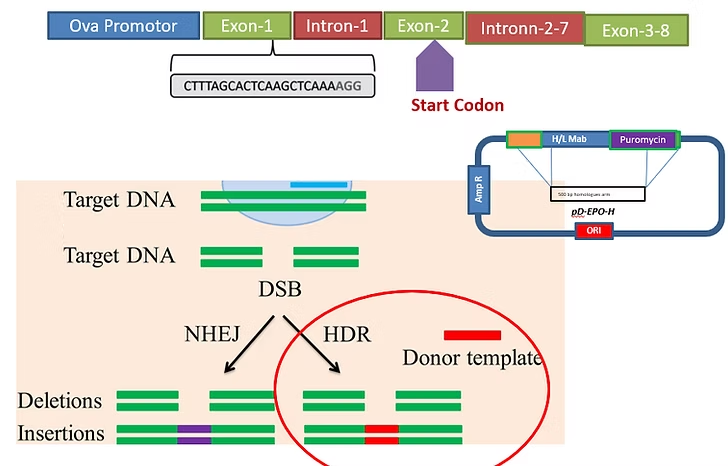
CRISPR technology enables precise genetic modifications in chicken PGCs, allowing targeted insertion of genes for valuable recombinant protein expression directly in egg production.
Scalable Bioreactor
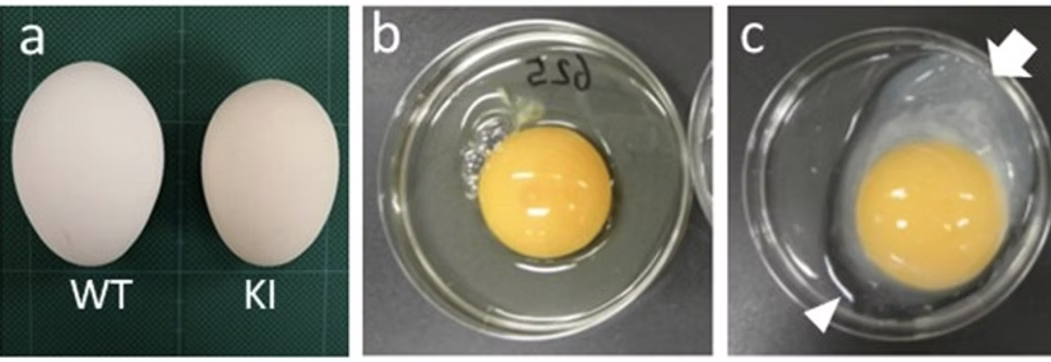
Each gene-edited chicken becomes a living bioreactor, producing designer eggs containing high-quality recombinant proteins. Simple, efficient, and infinitely scalable.